
Animals Are The Key — The virus that causes COVID-19 is not the same strain as what first emerged from China. A new study shows it has changed slightly in a way that makes it more contagious to humans.
Compared to the original strain, people infected with the new strain -- called 614G -- have higher viral loads in their nose and throat, though they don’t seem to get any sicker. But they are much more contagious to others.
“That kind of makes sense,” says Ralph Baric, PhD, a professor of epidemiology, microbiology, and immunology at the University of North Carolina at Chapel Hill.
The new strain has a change to its spike proteins -- the regions of its outer shell that dock on our cells and infect them. The change makes it a much more efficient predator. It passes quickly from cell to cell in our bodies, copying itself at a furious pace.
Baric’s experiments help to explain why the 614G strain, which first emerged in Europe in February, has quickly dominated worldwide spread. It is not the same as the current strain…— B1.1.7.
Bats More Than Likely Got Out Of The Belfry — He says the virus likely jumped out of bats and discovered a brand new population of human hosts, with more than 7 billion of us on the planet to infect. None of us has any immune defenses against it, so we are prime targets. Viruses with genetic advantages that help them copy themselves faster and jump more quickly between hosts are the versions that survive and will get passed on.
“So it can jump from person to person to person to person, that’s going to be the most competitive virus in terms of the virus maintaining itself,” says Baric, who is one of the world’s foremost experts on coronaviruses. His new study is published in the journal Science.
The new study backs up earlier research by a team of scientists led by Bette Korber, PhD, at Los Alamos National Laboratory in New Mexico. The team first noticed the rapid spread of the new strain and questioned whether the virus wasn’t evolving to become more easily passed between people.
In new experiments, animals infected with the new 614G strain passed it much more quickly to healthy animals than those infected with the original strain.
Sampling From China Of Bats — RaTG13 is the name, rank and serial number of an individual horseshoe bat of the species Rhinolophus affinis, or rather of a sample of its feces collected in 2013 in a cave in Yunnan, China. The sample was collected by hazmat-clad scientists from the Institute of Virology in Wuhan that year. Stored away and forgotten until January this year, the sample from the horseshoe bat contains the virus that causes Covid-19.
The scientists were mostly sampling a very similar species with slightly shorter wings, called Rhinolophus sinicus, in a successful search for the origin of the virus responsible for the SARS epidemic of 2002-03. That search had alarming implications, which were largely ignored.
In Shitou Cave, south of Kunming, the capital of Yunnan, they found viruses in the bats’ droppings and anal swabs that were more similar to human SARS than anything found in palm civets, the small mammals that until then were presumed to be the source of human infection. Back in the laboratory, they found that one of the viruses from bat droppings, called WIV1, could thrive in monkey and human cells specially engineered to activate the gene for ACE2 receptors, the lock to which a coronavirus’s spike protein can fit as a key. This suggested that people could catch SARS directly from a bat dropping.Fast-forward to the present day, Olival says what they found is alarming: “We found evidence for, in total, from all the sampling we did in China, about 400 new strains of coronaviruses.”
That means 400 potential candidates to spark another outbreak. After all, a coronavirus caused a massive outbreak in China back in 2002 — severe acute respiratory syndrome, or SARS. And this current outbreak is from a SARS-related coronavirus.
A direct path to humans, It gets worse: Scientists had thought spillovers were rare — that bat coronaviruses weren’t generally capable of infecting humans, so it took complicated steps.
Step one: A bat coronavirus would have to infect some animal species that had closer contact with people than bats do. Step two: While in that other animal's body, the virus would need to pick up new genetic code.
Bats Carry Many Viruses. So Why Don’t They Get Sick? — “What we showed was that SARS-related viruses in these bat populations have the potential to go directly into human cells and do not need that extra mutational step of infecting another host." In other words, the path to sparking new outbreaks is potentially much more direct.
For example, one of the coronaviruses that the researchers found was a very close genetic match for the SARS virus. So they put it in a petri dish with human cells. The virus succeeded in infecting the cells. Which brings us to this current coronavirus outbreak. As soon as it started, EcoHealth Alliance's longtime collaborators in China (principally researchers at the Wuhan Institute of Virology and the Wuhan Jinyintan Hospital) compared the new virus with the bat samples they'd collected. They found an extremely close match.
“A viral taxonomist would probably call that the same virus species," says Olival. That suggests this current outbreak — which has infected tens of thousands of people — could have come directly from bats, says Olival. And, he adds, the larger takeaway is clear: "These bat SARS-related coronaviruses are actively spilling over in the human population." Not all of them will spark deadly pandemics. But the more frequent these spillovers, the greater the chances.
Hamster Volunteers — Researchers at the University of Wisconsin in Madison infected 16 hamsters with the SARS-CoV-2 virus. Eight hamsters were infected with the new 614G strain. Eight others were infected with the original strain that was first identified in China. Each infected hamster was paired with a healthy hamster that was separated by a partition in a cage, so that the animals couldn’t touch but did breathe the same air.
By the second day of the experiment, five of the eight healthy hamsters sharing air with animals infected with the 614G strain had fallen ill and were shedding the virus themselves, but none of the healthy hamsters paired with those infected with the original strain had gotten sick.
The original strain did eventually sicken the healthy animals, but it took 2 more days for that to happen, proving that the changes helped to speed the spread of the virus
Baric and his team also wondered if the changes to the structure of the virus would affect how future therapies -- including a vaccine -- might work against it, since all the treatments now in development have been designed to counter the original strain that emerged from China.
They tested antibodies extracted from the blood of people who had survived COVID-19 infections on both the new and old strains, and they found no significant differences in how well those antibodies worked to neutralize the virus.
That’s good news, because it means people who recover from an infection with the original strain might still have some protection against the new strain.
In the U.S., the original strain was imported from China and began circulating on the West Coast, while the new strain was imported from Europeans who were mainly traveling to New York and the rest of the East Coast.
How This Might End: Lessons From the Spanish Flu — Baric and his team also tested the antibodies that are being developed as treatments to give people passive immunity against the virus. Those seemed to work well, too. “The vaccines, which are all based on the original Chinese strain, make a good immune response that protects against this strain, so that’s good news,” he says.
While current treatments and prevention efforts don’t seem to be affected much by this change to the virus, the mutation does raise questions about how fast new strains are emerging and whether or not one of those might cause a problem in the future, Baric says.
Coronaviruses, as a group, are extremely stable. They have a special molecule — rightly dubbed a proofreader -- that makes sure the virus gets copied correctly. Because of this proofreader, the speed of the emergence of these new strains of the new COVID-19 has been somewhat surprising to scientists who study them.
The Mink Might Be A Carrier — Is indicated — 
The American mink is at home both on land and in water—it often makes its burrow along stream banks.
The American mink is a fascinating animal with a vast range that encompasses most of North America, except for Arizona, along the Arctic coast, and on some offshore islands.
Moving with ease between land and water, the American mink usually chooses to live in woodsy areas near streams, ponds, or lakes, creating its burrow in the banks of a river, stream, or lake, or sometimes appropriating an abandoned den of a muskrat. Their ideal habitat also includes brushy cover or rocky crevices.
Roughly half of the mink’s length is its tail. Its lanky body has short legs, and due to its semi-aquatic ways, its toes are partially webbed. Males are both heavier and longer than females, by about 20 percent, with the smallest male being roughly the size of the largest female: about 2.5 pounds and 23 inches. Their slender form enables them to maneuver with speed and agility both when pursuing prey and escaping predators.
The American mink has been -- and continues to be -- exploited for its exceptionally beautiful and soft fur. Most states and all Canadian provinces continue to have trapping seasons. Habitat loss is another significant threat to the American mink, as it depends on aquatic areas, which are often developed or altered in other damaging ways. Improper disposal of chemicals also takes a toll on the mink’s reproduction and health.
Humane Society Wildlife Land Trust wildlife sanctuaries, such as the Kullberg Memorial Wildlife Sanctuary in Swanzey, New Hampshire, and the Orenda-Winham Wildlife Sanctuary and Stickey Wicket Wildlife Sanctuary, both in Marlow, New Hampshire, offer the American mink healthy habitat where it is protected against the commercial and recreational trapping that threaten its safety elsewhere.
American mink are known to be fierce predators, and they boldly defend themselves against such formidable predators as coyotes, bobcats, and birds of prey. Among their ways of avoiding predation are their secretive nature, coloring that confuses the eye, agility when trying to escape, and confining most activity between dusk and dawn, when their coloring makes them even less visible. The prey they seek for themselves includes crayfish, small frogs, small mammals, fish, ducks, and other waterfowl.
As far as pets go… not a good idea unless you value your fingers and know a good surgeon…
Bottom Line Is not Good for Minks In Captivity — One development that Baric and other scientists are closely watching is the emergence of new strains found on mink farms in Denmark and the Netherlands that have been shown to infect humans. There’s work being done to confirm that at least one of those strains — the so-called cluster 5 virus — may have evolved enough changes to its spike proteins that help it escape the vaccine.
Baric says the research needs to be verified, but early work suggests that the virus appears to have changed to help it infect minks more efficiently, while also keeping its ability to infect humans.
When a virus evolves in a way that allows it to circulate in an animal species, “it becomes more difficult to eradicate that virus,” he says. Baric says if the virus continues to be passed in minks, if we vaccinated everyone in Denmark, but left the minks, the virus would hang out until there were enough new, susceptible hosts — typically young children — and then jump back into humans.
For that reason, he says mink farms may need to take further steps, like vaccinating their animals, or, in the worst case, killing their minks, to control the spread.
No Mas, No Mas ! — As COVID-19 vaccination rates pick up around the world, people have reasonably begun to ask: how much longer will this pandemic last? It’s an issue surrounded with uncertainties. But the once-popular idea that enough people will eventually gain immunity to SARS-CoV-2 to block most transmission — a ‘herd-immunity threshold’ — is starting to look unlikely.
That threshold is generally achievable only with high vaccination rates, and many scientists had thought that once people started being immunized en masse, herd immunity would permit society to return to normal. Most estimates had placed the threshold at 60–70% of the population gaining immunity, either through vaccinations or past exposure to the virus. But as the pandemic enters its second year, the thinking has begun to shift.
In February, independent data scientist Youyang Gu changed the name of his popular COVID-19 forecasting model from ‘Path to Herd Immunity’ to ‘Path to Normality’. He said that reaching a herd-immunity threshold was looking unlikely because of factors such as vaccine hesitancy, the emergence of new variants and the delayed arrival of vaccinations for children.
Gu is a data scientist, but his thinking aligns with that of many in the epidemiology community. “We’re moving away from the idea that we’ll hit the herd-immunity threshold and then the pandemic will go away for good,” says epidemiologist Lauren Ancel Meyers, executive director of the University of Texas at Austin COVID-19 Modeling Consortium. This shift reflects the complexities and challenges of the pandemic, and shouldn’t overshadow the fact that vaccination is helping. “The vaccine will mean that the virus will start to dissipate on its own,” Meyers says.
But as new variants arise and immunity from infections potentially wanes, “we may find ourselves months or a year down the road still battling the threat, and having to deal with future surges”.
Long-term prospects for the pandemic probably include COVID-19 becoming an endemic disease, much like influenza. But in the near term, scientists are contemplating a new normal that does not include herd immunity. Here are some of the reasons behind this mindset, and what they mean for the next year of the pandemic.
It’s Unclear Whether Vaccines Prevent Transmission — The key to herd immunity is that, even if a person becomes infected, there are too few susceptible hosts around to maintain transmission — those who have been vaccinated or have already had the infection cannot contract and spread the virus. The COVID-19 vaccines developed by Moderna and Pfizer–BioNTech, for example, are extremely effective at preventing symptomatic disease, but it is still unclear whether they protect people from becoming infected, or from spreading the virus to others. That poses a problem for herd immunity.
The COVID-19 Is Here To Stay — Here’s What That Means — “Herd immunity is only relevant if we have a transmission-blocking vaccine. If we don’t, then the only way to get herd immunity in the population is to give everyone the vaccine,” says Shweta Bansal, a mathematical biologist at Georgetown University in Washington DC. Vaccine effectiveness for halting transmission needs to be “pretty darn high” for herd immunity to matter, she says, and at the moment, the data aren’t conclusive. “The Moderna and Pfizer data look quite encouraging,” she says, but exactly how well these and other vaccines stop people from transmitting the virus will have big implications.
A vaccine’s ability to block transmission doesn’t need to be 100% to make a difference. Even 70% effectiveness would be “amazing”, says Samuel Scarpino, a network scientist who studies infectious diseases at Northeastern University in Boston, Massachusetts. But there could still be a substantial amount of virus spread that would make it a lot harder to break transmission chains
.
Vaccine Roll-Out Is Uneven — The speed and distribution of vaccine roll-outs matters for various reasons, says Matt Ferrari, an epidemiologist at Pennsylvania State University’s Center for Infectious Disease Dynamics in University Park. A perfectly coordinated global campaign could have wiped out COVID-19, he says, at least theoretically. “It’s a technically feasible thing, but in reality it’s very unlikely that we will achieve that on a global scale,” he says. There are huge variations in the efficiency of vaccine roll-outs between countries (see ‘Disparities in distribution’), and even within them.
Variant Information — For example, some variant viruses are of particular concern because they spread easier, cause more severe disease, or may escape the body’s immune response.
As CDC and public health partners sequence more SARS-CoV-2 genomes, we will improve our understanding of which variants are circulating in the US, how quickly variants emerge, and which variants are the most important to characterize and track in terms of health.
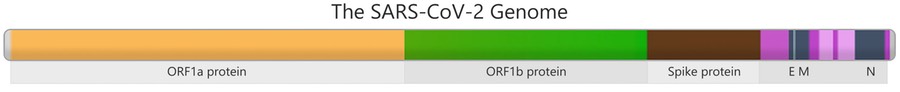
Genes in the SARS-CoV-2 genome that contain instructions to build parts of the virus are shown in different colors. For example, the brown section in the picture has genetic instructions to build the Spike protein, which then allows the virus to attach to human cells during infection. This section of the genome serves as a key region for monitoring mutations.

How Do Variants Occur?
The virus genome is packed inside an envelope that contains proteins, including the Spike protein.
Mutations are changes in the genetic code of a virus that naturally occur over time when an animal or person is infected. While a certain amount of genetic variation is expected to occur as SARS-CoV-2 spreads, it’s important to monitor circulating viruses for key mutation(s) that happen in important regions of the genome.
Many mutations do not affect the virus’s ability to spread or cause disease because they do not alter the major proteins involved in infection; eventually these are outcompeted by variants with mutations that are more beneficial for the virus.
What is CDC doing to track SARS-CoV-2 variants — In the United States, CDC tracks emerging variants through genomic surveillance with the following approaches:
🏥 Virus Solid Icon — Leading the National SARS-CoV-2 Strain Surveillance (NS3) system: — Since November 2020, CDC regularly receives SARS-CoV-2 samples from state health departments and other public health agencies for sequencing, further characterization, and evaluation. On January 25, 2021, the NS3 system was scaled-up to process 750 samples per week. Notable strength of this system is the regular collection of numerous representative specimens from across the country and characterization of viruses beyond what sequencing alone can provide.
🏥 Lab Icon — Partnering with commercial diagnostic laboratories
CDC is contracting with large commercial diagnostic labs to sequence samples across the United States. CDC has commitments from these laboratories to sequence 6,000 samples per week, with the capacity to scale up in response to the nation’s needs.
🏥 Landmark Icon — Collaborating with universities:
CDC has contracts with seven universities to conduct genomic surveillance research in collaboration with public health agencies. The studies are meant to provide deeper insights into viral genomics and molecular epidemiology within the various regions across the country.
🏥 USA Map Icon — Supporting State, territorial, local and tribal health departments
Since 2014, CDC’s Advanced Molecular Detection Program has been integrating next-generation sequencing and bioinformatics capabilities into the US public health system. Several state and local health departments have been applying these resources as part of their response to COVID-19. Public health departments support local investigations, conduct studies, and make genomic data available to public databases. To further support these efforts, on December 18, 2020, CDC released $15 million from COVID supplemental funds through the Epidemiology and Laboratory Capacity Program.
![]()
🏥 Leading the SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology, and Surveillance (SPHERES) consortium - Since early in the pandemic, CDC has led a national consortium of laboratories sequencing SARS-CoV-2 (SPHERES). The SPHERES consortium consists of more than 160 institutions, including academic centers, industry, non-governmental organizations, and public health agencies. Through this coordination, genomic data are made available through public databases for use by public health professionals, researchers, and industry.
Why Is Genomic Surveillance Important For Public Health?
Routine analysis of genetic sequence data enables CDC and its public health partners to identify and characterize variant viruses—either new ones identified here or those already identified abroad—and to investigate how variants impact COVID-19 disease severity and how variants impact the effectiveness of vaccines and therapeutics.
Surveillance of emerging variants can help detect variant with:
- Ability to spread more quickly in people
- Ability to cause either milder or more severe disease in people
- Ability to evade detection by specific diagnostic tests
Many commercial nucleic acid amplification tests that use reverse transcription polymerase chain reaction (RT-PCR) have multiple targets to detect the virus, such that even if a mutation impacts one of the targets, the other RT-PCR targets will still work. - However, there are some tests that rely on only one target, and mutations may impact their ability to work. FDA is using public health sequencing data to monitor mutations and their impact on confidential/proprietary diagnostic test designs.
- Decreased susceptibility to therapeutics that employ monoclonal antibodies
Such therapy involves specifically designed antibodies that target regions of the virus to block infection. Because these treatments are more specific than natural immune response-generated antibodies, they may be less effective against variants that emerge. - Ability to evade natural or vaccine-induced immunity
Both natural infection with and vaccination against SARS-CoV-2 produce a “polyclonal” antibody response that targets several parts of the spike protein. The virus would need to accumulate significant mutations in the spike protein to evade immunity induced by vaccines or by natural infection.
Among these possibilities, the ability to evade vaccine-induced immunity would be the most concerning. There is no definitive evidence yet that this is occurring, but scientists are closely evaluating this possibility.
HOW THE PANDEMIC CAME ABOUT
BY CHARLIE CAMPBELL, YUXI, YUNNAN, AND ALICE PARK… Brilliant Work 07/23/2020
It wasn’t greed, or curiosity, that made Li Rusheng grab his shotgun and enter Shitou Cave. It was about survival. During Mao-era collectivization of the early 1970s, food was so scarce in the emerald valleys of southwestern China’s Yunnan province that farmers like Li could expect to eat meat only once a year–if they were lucky. So, craving protein, Li and his friends would sneak into the cave to hunt the creatures they could hear squeaking and fluttering inside: bats.
Li would creep into the gloom and fire blindly at the vaulted ceiling, picking up any quarry that fell to the ground, while his companions held nets over the mouth of the cave to snare fleeing bats. They cooked them in the traditional manner of Yunnan’s ethnic Yi people: boiled to remove hair and skin, gutted and fried. “They’d be small ones, fat ones,” says Li, now 81, sitting on a wall overlooking fields of tobacco seedlings. “The meat is very tender. But I’ve not been in that cave for over 30 years now,” he adds, shaking his head wistfully. “They were very hard times.”
China today bears little resemblance to the impoverished nation of Li’s youth. Since Deng Xiaoping embraced market reforms in 1979, the Middle Kingdom has gone from strength to strength. Today it is the world’s No. 2 economy and top trading nation. It has more billionaires than the U.S. and more high-speed rail than the rest of the world combined. Under current strongman President Xi Jinping, China has embarked on a campaign to regain “center place in the world.” Farmers like Li no longer have to hunt bats to survive.
Shitou Cave — That doesn’t mean
Shitou Cave has faded in significance. Today, though, its musty depths speak
not to local sustenance but global peril. Shitou was where Shi Zhengli, lead
scientist at the Wuhan Institute of Virology (WIV), working with samples of bat
feces in 2011 and 2012, isolated a novel virus that was very similar to SARS,
which had been responsible for a pandemic a decade earlier. Shi–known as
China’s “bat woman” for her tireless research on the winged mammal–warned that
other bat-borne diseases could easily spill over into human populations again.
Seven years later, her fears appear vindicated. In a February paper, Shi
revealed the discovery of what she called the “closest relative” of what would
become known as SARS-CoV-2, the coronavirus that causes COVID-19. It also
originated in Shitou Cave.
Dubbed RaTG13, Shi’s virus has a 96.2% similarity with the virus that has claimed some 600,000 lives across the world, including more than 140,000 in the U.S. Shi’s discovery indicates COVID-19 likely originated in bats–as do rabies, Ebola, SARS, MERS, Nipah and many other deadly viruses.
But how did this virus travel from a bat colony to the city of Wuhan, where the coronavirus outbreak was first documented? And from there, how did it silently creep along motorways and flight routes to kill nurses in Italy, farmers in Brazil, retirees in Seattle? How this virus entered the human population to wreak such a devastating toll is the foremost issue of global scientific concern today.
The search for “patient zero”–or the “index case,” the first human COVID-19 infection–matters. Not because any fault or blame lies with this individual, but because discovering how the pathogen entered the human population, and tracing how it flourished, will help the science and public-health communities better understand the pandemic and how to prevent similar or worse ones in the future.
On top of the millions of lives that hang in the balance, Cambridge University puts at $82 trillion across five years the cost to the global economy of the current pandemic. The human race can ill afford another.
The Provenance of COVID-19 — Is not only a scientific question. The Trump Administration also regards it as a political cudgel against Beijing. As the U.S. has failed to control outbreaks of the coronavirus and its economy founders, President Donald Trump has deflected blame onto China.
Trump and senior Administration figures have dubbed COVID-19 the “China virus” and “Wuhan virus.” Secretary of State Mike Pompeo said there was “enormous evidence” the virus had escaped from Shi’s lab in the city. (He has yet to share any hard evidence.) “This is the worst attack we’ve ever had on our country. This is worse than Pearl Harbor. This is worse than the World Trade Center,” Trump said in May of the pandemic, pointing the finger at China. In response, Chinese Foreign Minister Wang Yi accused the U.S. President of trying to foment a “new cold war” through “lies and conspiracy theories.”
The origin of the virus is clearly a touchy subject. Nevertheless, the world desperately needs it broached. Australia and the E.U. have joined Washington’s calls for a thorough investigation into the cause of the outbreak. On May 18, Xi responded to pressure to express support for “global research by scientists on the source and transmission routes of the virus” overseen by the World Health Organization.
But Trump has already accused the WHO of being “Chinacentric” and vowed to stop funding it. His attacks may have some basis in fact. The organization refused self-governing Taiwan observer status under pressure from Beijing. And privately, WHO officials were frustrated by the slow release of information from the Chinese authorities even as they publicly praised their transparency, according to transcripts obtained by the Associated Press.
Partisan bickering and nationalism threaten to eclipse the invaluable scientific work required to find the true source of the virus. Time is of the essence; a SARS vaccine was within touching distance when research that could have proved invaluable today was discontinued as the crisis abated. “Once this pandemic settles down, we’re going to have a small window of opportunity to put in place infrastructure to prevent it from ever happening again,” says Dr. Maureen Miller, a Columbia University epidemiologist.
The search for the virus’s origins must begin behind the squat blue-shuttered stalls at Wuhan’s Huanan seafood market, where the outbreak of viral pneumonia we now know as COVID-19 was first discovered in mid-December. One of the first cases was a trader named Wei Guixian, 57, who worked in the market every day, selling shrimp out of huge buckets. In mid-December she developed a fever she thought was a seasonal flu, she told state-run Shanghai-based the Paper. A week later, she was drifting in and out of consciousness in a hospital ward.
The Epidemic Grows — Of the first 41 patients hospitalized in Wuhan, 13 had no
connection to the marketplace, including the very first recorded case. That
doesn’t necessarily excuse the market as the initial point of zoonotic jump,
though–we don’t know yet for certain how many COVID-19 cases are asymptomatic,
but research suggests it could be as high as 80%. And, even if Huanan market
wasn’t where the virus first infected humans, it certainly played a huge role
as an incubator of transmission. At a Jan. 26 press conference, the Hong Kong Centre
for Health Protection revealed 33 of 585 environmental samples taken after the
market was shut Jan. 1 tested positive for the virus. Of these, 31 were taken
in the western section where wildlife was sold.
In May, China acceded to demands for an independent inquiry after more than 100 countries supported a resolution drafted by the E.U. Still, President Xi insists it must be “comprehensive”–looking not just at China but also at how other nations responded to the WHO’s warnings–and cannot begin until after the pandemic has subsided. “The principles of objectivity and fairness need to be upheld,” Xi told the World Health Assembly. (Notably, inquiries into the 2009 H1N1 “swine flu” pandemic and 2014 West African Ebola outbreak began before the crises had abated.) According to past investigations’ protocols, teams are composed of independent public-health experts and former WHO staff appointed by the WHO based on member states’ recommendations. At a practical level, however, any probe within China relies on cooperation from Beijing, and it’s uncertain whether the U.S. will accept the findings of a body Trump has slammed for “severely mismanaging and covering up the spread of the coronavirus.Peter Ben Embarek, a food-safety and animal-disease expert at the WHO, says an investigation must concentrate on interviews with all the initial cases, trying to find clues about potential earlier infections among their relatives, their contacts, and where they had been over the days and weeks before they got sick. Also, which hunters and farmers supplied what species of animals. “With a bit of luck and good epidemiological work, it can be done,” he says.
There Are Many Who Look — At where COVID-19 emerged and see something that can’t be just a co-incidence. In 2017, China minted its first biosecurity-level 4 (bsl-4) laboratory–the highest level cleared to even work with airborne pathogens that have no known vaccines–in Wuhan. Ever since, the country’s foremost expert on bat viruses has been toiling away inside the boxy gray buildings of the WIV. Indeed, when Shi first heard about the outbreak, she herself thought, “Could they have come from our lab?” she recently told Scientific American. An inventory of virus samples reassured her that it hadn’t, she added, yet that hasn’t stopped some from maintaining their suspicions.
Mistakes do happen. The last known case of small-pox leaked from a U.K. laboratory in 1978. SARS has leaked from Chinese laboratories on at least two occasions, while U.S. scientists have been responsible for mishandlings of various pathogens, including Ebola. There are only around 70 bsl-4 laboratories in 30 countries. Suspicions regarding the nature of research under way inside the Wuhan laboratory persist. According to one leading virologist, who asked to remain anonymous for fear of jeopardizing funding and professional relationships, “Were you to ask me where’s the most likely place in the world for a naturally occurring bat coronavirus to escape from a laboratory, Wuhan would be in the top 10.”
Still, neither the WHO nor the Five Eyes intelligence network–comprising the U.S., U.K., Canada, Australia and New Zealand–has found evidence that COVID-19 originated from Shi’s lab. Canberra has even distanced itself from a U.S.-authored dossier that sought to convince the Australian public that the Five Eyes network had intelligence of a Chinese cover-up. (It appeared to rely exclusively on open-source material.) Meanwhile, scientific peers have rallied to defend Shi from suspicion. “She is everything a senior scientist should be,” says Miller, who has collaborated with Shi on various studies. The Wuhan Institute of Virology did not respond to requests for comment.
Available evidence suggests COVID-19 leaped from wild animal to human. Tracing exactly how is crucial. It enables governments to install safeguards regarding animal husbandry and butchery to prevent any repeat. SARS, for example, originated in bats and then infected a palm civet, a catlike mammal native to South and Southeast Asia. The animal was then sold at a wet market–where fresh meat, fish and sometimes live animals are sold–in Guangdong, from which it jumped to humans. In the wake of that outbreak, which claimed at least 774 lives worldwide, palm civets were banned from sale or consumption in China.
Bats may have been the initial reservoir for SARS-CoV-2, but it’s likely that there was an intermediary before it got to humans, and that’s where the possibilities grow. Bats share Shitou Cave with starlings, for one, and at least one large white owl nests in its upper reaches. Herds of black and white goats graze the dusty shrub all around the cave opening, while the Yi ethnic group traditionally rear and eat dogs. Bat guano is also traditionally prized as a fertilizer on crops.
Just a Few Miles From Shitzu — Customers at Baofeng Horse Meat restaurant squat by round tables, slurping green tea poured from enormous brass teapots, while charcoal burners cook up the eponymous cuts alongside dogmeat and other specialties. “All the animals we sell are reared nearby,” says proprietor Wang Tao. Cultural practices and disease-transmission vectors are often entwined. MERS continues to jump between camels and their human handlers on the Arabian Peninsula. China’s penchant for eating rare and unusual wildlife for obscure health benefits may have contributed to the current pandemic.
While many aspects of Traditional Chinese Medicine (TCM) are entirely benign, involving little more than massage, pressure points and bitter herbs, there is a fetishization of exotic animals, and there’s some evidence that TCM might have played a role in launching the pandemic. The receptor-binding domain of SARS-CoV-2’s spike protein–which the virus uses to bind to hosts–is unusually adept at attaching to human cells.
New viruses discovered in Malaysian pangolins have since been shown to have exactly the same receptor binders. “Some features in [SARS-CoV-2] that initially may have looked unusual, you’re now finding in nature,” says Edward Holmes, an evolutionary biologist and virologist at the University of Sydney.
That COVID-19 originated in bats and then jumped to humans via a pangolin intermediary is now the most likely hypothesis, according to multiple studies (although some virologists disagree). Up to 2.7 million of the scaly mammals have been plucked from the wild across Asia and Africa for consumption mostly in China, where many people believe their scales can treat everything from rheumatoid arthritis to inflammation. Their meat is also highly prized for its supposed health benefits.
On Feb. 24, China announced a permanent ban on wildlife consumption and trade, scratching out an industry that employs 14 million people and is worth $74 billion, according to a 2017 report commissioned by the Chinese Academy of Engineering. It’s again extremely sensitive. President Xi is an ardent supporter of TCM and has promoted its use globally. The total value of China’s TCM industry was expected to reach $420 billion by the end of this year, according to a 2016 white paper by China’s State Council.
And rather than raising the possibility that misuse of TCM sparked the outbreak, Chinese state media has lauded–without evidence–the “critical role” TCM has played in the treatment of COVID-19 patients. In an apparent attempt to head off criticism related to the pandemic, draft legislation was published in late May to ban any individual or organization from “defaming” or “making false or exaggerated claims” about TCM. Cracking down on the illicit animal trade would go a long way toward preventing future outbreaks. But as demand for meat grows across increasingly affluent Asia, Africa and Latin America, the potential for viruses to spill over into human populations will only increase.
It probably wasn’t blind luck that Li and his friends didn’t get sick from their hunting expeditions in Shitou Cave. Research by Columbia’s Miller with WIV’s Shi, published in 2017, found that local people were naturally resistant to SARS-like viruses. Examining their lifestyle habits and antibodies can help deduce both mitigating factors and possible therapies, while pinpointing which viruses are particularly prone to infecting humans, potentially allowing scientists to design vaccines in advance. “They are the canaries in the coal mine,” says Miller.
The Cloud Of Uncertainty Surrounding The Virus’s Origins — May never lift. Identifying an individual “patient zero” where the virus made the jump from animal to human may be rendered impossible by its remarkable ability to spread while asymptomatic. But just as important is uncovering the broader map of how the virus spread and changed genetically as it did so. In theory, that sort of genetic surveillance could foster the development of broad-spectrum vaccines and antivirals that may prove effective against future novel outbreaks. Studying the anatomy of viruses that readily jump between species may even help predict where the next pandemic is coming from, and prepare us for the inevitable next time. So did those of his 40-member team of infectious-disease emergency responders at Providence Regional Medical Center in Everett, Wash. The first time, the alert was part of a routine monthly test. This time, it was the real thing.
The page signaled the first confirmed U.S. case of COVID-19. The patient was a Washington State resident who had recently returned from visiting family in Wuhan, where the disease was spreading rapidly. Aware of his higher risk, and concerned when he developed a fever, the 35-year-old (who wishes to remain anonymous) visited an urgent-care center where he told health care providers about his travel history. They notified the state health department, which in turn helped the care center send a sample for testing to the Centers for Disease Control and Prevention (CDC) in Atlanta–at the time, the only labs running COVID-19 tests. When the test was positive, CDC scientists recommended the patient be hospitalized for observation. And Diaz’s team was paged.
A trained ambulance team arrived at the man’s home, moved him into a specially designed mobile isolation unit, and drove 20 minutes to Providence Regional. There, the patient couldn’t see who greeted him; everyone assigned to his care was garbed in layers of personal protective equipment. Once in his room, he spoke to medical staff only through a tele-health robot equipped with a screen that displayed their faces, transmitted from just outside the room.
A nurse carefully swabbed the back of his nose and pharynx for a sample of the virus that had brought him to the hospital. Not only was he the first confirmed case of COVID-19 in the U.S., he was also the first in the country to have his virus genetically sequenced. As the index patient in the U.S., his sequence, named WA1 (Washington 1), served as the seed from which experts would ultimately trace the genetic tree describing SARS-CoV-2’s path from person to person across communities, countries and the globe, as it mutated and either died out or moved on with renewed vigor to infect more people.
Genetic sequencing is
a powerful tool to combat viruses’ fondness for mutating. Viruses are
exploitative and unscrupulous; they don’t even bother investing in any of their
own machinery to reproduce. Instead, they rely on host cells to do that–but it
comes at a price. This copying process is sloppy, and often leads to mistakes,
or mutations. But viruses can sometimes take advantage of even that; some
mutations can by chance make the virus more effective at spreading undetected
from host to host. SARS-CoV-2 seems to have landed on at least one such suite
of genetic changes, since those infected can spread the virus even if they
don’t have any symptoms.
Figuring out how to map those changes is a fairly new science. Following the
2014 West African outbreak of Ebola, scientists mapped the genomes of about
1,600 virus samples, collected from the start of the outbreak and representing
about 5% of total cases. The work offered insights into how Ebola moved between
locations and mutated. But it wasn’t published until 2017, because the majority
of the sequencing and sharing of that data was done after the disease’s peak,
says Trevor Bedford, associate professor at the Fred Hutchinson Cancer Research
Center and co-founder of Nextstrain.org, an open-source database of
SARS-CoV-2 genetic sequences. With COVID-19, “ Everything is happening much
more quickly,” he says, which makes the information more immediately useful.
Since The First Sars-Cov-2 Genome Was Published And — Made publicly available online in January, scientists have mapped the genomes of over 70,000 (and counting) samples of the virus, from patients in China, the US the E.U, Brazil and South Africa, among others. They deposited those sequences into the Global Initiative on Sharing All Influenza Data (GISAID), a publicly available genetic database created in 2008 initially to store and share influenza genomes. During the coronavirus pandemic, it has quickly pivoted to become a clearinghouse for tracking the genetics of SARS-CoV-2, enabling scientists to map the virus’s march across continents and detail its multipronged attack on the world.
“We have genomes from researchers and public-health labs from all over the world on six continents,” says Joel Wertheim, associate professor of medicine at University of California, San Diego. “It provides us with unique insight and confidence that other types of epidemiological data just cannot supply.” Relying on the GISAID sequences, Nextstrain has become a virtual watering hole for scientists–and increasingly public-health officials–who want to view trends and patterns in the virus’s genetic changes that can help inform decisions about how to manage infections.
If genetic sequencing is the new language for managing infectious-disease outbreaks, then the mutations that viruses generate are its alphabet. If paired with information on how infected patients fare in terms of their symptoms and the severity of their illness, genomic surveillance could reveal useful clues about which strains of virus are linked to more severe disease. It might shed light on the mystery of why certain victims of the virus are spared lengthy hospital stays and life-threatening illness. As nations start to reopen, and before a vaccine is widely available, such genetic intel could help health care providers to better plan for when and where they will need intensive-care facilities to treat new cases in their community.
Genetic information is also critical to developing the most effective drugs and vaccines. Knowing the sequence of SARS-CoV-2 enabled Moderna Thera-peutics to produce a shot ready for human testing in record time: just two months from when the genetic sequence of SARS-CoV-2 was first posted. Even after a vaccine is approved and distributed, continuing to track genetic changes in SARS-CoV-2 to ensure it’s not mutating to resist vaccine-induced immunity will be critical. The data collected by Nextstrain will be crucial to help vaccine researchers tackle mutations, potentially for years to come. Already, the group advises the WHO on the best genetic targets for the annual flu shot, and it plans to do the same for COVID-19. “We can track the areas of the virus targeted by the vaccine, and check the mutations,” says Emma Hodcroft from the University of Basel, who co-developed Nextstrain. “We can predict how disruptive those mutations are to the vaccine or not and tell whether the vaccines need an update.”
Meanwhile, genetic surveillance provides real-time data on where the virus is going and how it’s changing. “This is the first time during an outbreak that lots of different researchers and institutes are sharing sequencing data,” says Barbara Bartolini, a virologist at the Lazzaro Spallanzani National Institute for Infectious Diseases in Rome, who has sequenced dozens of viral samples from patients in Italy. That information is giving public-health experts more precise information on the whereabouts of its viral enemy that no traditional disease-tracking method can supply.
After Diaz’s patient tested positive for SARS-CoV-2, Washington State public-health officials diligently traced the places the patient had been and the people he’d come in contact with. He had taken a ride-share from the airport, gone to work and enjoyed lunch at a seafood restaurant near his office with colleagues. But because so little was known about the virus at the time, these contact tracers were focusing mostly on people with symptoms of illness–and at the time, none of the patient’s contacts reported them. The genetics, however, told a different story.
Seattle Happened To Have Launched A Program In 2018 To Track Flu Cases —By collecting samples from patients in hospitals and doctors’ offices, sites on college campuses, homeless shelters, the city’s major international airport and even from volunteers with symptoms who agreed to swab their nasal passages at home. Those that were positive for influenza and other respiratory illnesses had their samples genetically sequenced to trace the diseases’ spread in the community. As COVID-19 began to emerge in the Seattle area at the end of February, Bedford and his colleagues began testing samples collected in this program for SARS-CoV-2, regardless of whether people reported symptoms or travel to China, then the world’s hot spot for the virus. That’s how they found WA2, the first case in Washington that wasn’t travel-related. By comparing samples from WA1, WA2 and other COVID-19 cases, they figured out that SARS-CoV-2 was circulating widely in the community in February.
If that community-based sequencing work had been conducted earlier, there’s a good chance it might have picked up cases of COVID-19 that traditional disease-tracking methods, which at the time focused only on travel history and symptoms, missed. That would have helped officials make decisions about a lockdown sooner, and might have helped to limit spread of the virus. SARS-CoV-2 moves quickly but mutates relatively slowly, for a virus–generating only about two mutations every month in its genome. For drug and vaccine developers, it means the virus can still evade new treatments designed to hobble it. Those same changes serve as passport stamps for its global trek through the world’s population, laying out the itinerary of the virus’s journey for geneticists like Bedford. The cases in the initial Seattle cluster, he says, appear to have all been connected, through a single introduction directly from China to the U.S. in mid- to late January. Until the end of February, most instances of SARS-CoV-2 in the U.S. piggybacked on unwitting travelers from China. But as the pandemic continued, that changed.
Genetic analysis confirmed that on Feb. 26, SARS-CoV-2 had already hit a new milestone, with the first documented case that it had successfully jumped to a new host in Santa Clara, Calif., one with no travel history to the infectious-disease hot spots in China or known contact with anyone who had traveled there. It’s not clear how this person got infected, but genetic sequencing showed this patient passed on the virus to two health care workers while being treated in the hospital–and that the virus was already spreading in the community, without help from imported cases.
Bedford’s team began to see mutations in samples from Seattle that matched samples from people in Europe and the U.S.’s East Coast. “At the beginning we could kind of draw a direct line from viruses circulating in China to viruses circulating in the Seattle area,” says Bedford. “Later, we see that viruses collected from China have some mutations that were seen later in Europe, and those same mutations were seen in viruses in New York. So, we can draw another line from China to Europe to New York” and then on to Seattle. The virus had begun multiple assaults into the US
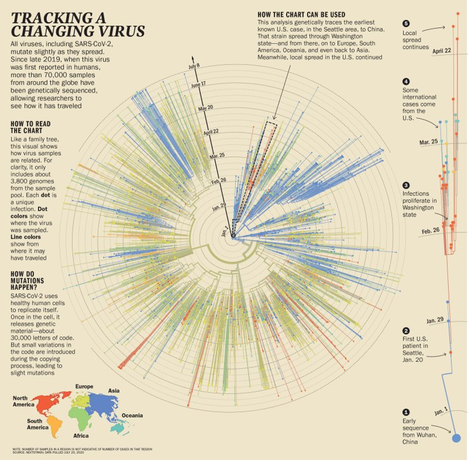
Around The World, Virologists Were Seeing Similar Stories — Written in the genes of SARS-CoV-2. In January, a couple from Hubei province arrived in Rome, eager to take in the sights of the historic European city. By Jan. 29, they were hospitalized at Lazzaro Spallanzani National Institute for Infectious Diseases with fever and difficulty breathing. Tests confirmed they were positive for SARS-CoV-2.
Bartolini, a virologist at the hospital, and her colleagues compared the genetic sequences from a sample taken from the wife to sequences posted on GISAID. The Italian researchers found it matched five other samples from patients as far-flung as France, Taiwan, the U.S. and Australia. SARS-CoV-2 was clearly already on a whirlwind tour of the planet.
Not all strains of SARS-CoV-2 are equally virulent; some branches of its genetic tree are likely to grow larger and sprout further offshoots, while others terminate more quickly, says Harm van Bakel, assistant professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai. His team conducted the first genetic sequencing analysis of cases in New York City, which quickly became a U.S. hot spot; by March the city had seen a half a dozen or so separate introductions of SARS-CoV-2, but only two resulted in massive spread of the virus. The remainder petered out without transmitting widely.
Retrospectively, there’s no way to tell for sure if these two strains were simply in the right place at the right time–in a particularly densely populated area of the city, for example, or in an area where people congregated and then dispersed to other parts of the city–or if they were actually more infectious. But determining the genetic code of a circulating virus early may help scientists and governments decide which strains are worth worrying about and which aren’t.
From analyzing genetic sequences from 36 samples of patients in Northern California, Dr. Charles Chiu, professor of laboratory medicine and infectious diseases at the University of California, San Francisco, says it might have been possible to identify the major circulating strains and track how they spread if more testing were available to know who was infected–and use this information to guide quarantine and containment practices. “There was a window of opportunity that if we had more testing and more contact-tracing capacities available early on, we likely would have prevented the virus from gaining a foothold at least in California,” he says.
There were similar missed opportunities in Chicago, where genetic sequencing of 88 viruses revealed that the outbreak resulted from three main strains. One was similar to those circulating in New York; one was closely related to the Washington cases and a third never spread appreciably outside the Chicago area. This suggests that stricter travel restrictions might have helped limit introduction of the virus and transmission in northern Illinois.
Ongoing Genetic Sequencing Can Also Help Officials Tailor Narrower Strategies to quell the spread of a virus. It wasn’t long after Beijing reopened following two months of lockdown that infections began creeping up again in June. Sequencing of the new cases revealed that the viruses circulating at the time shared similarities with viruses found in patients in Europe, suggesting the cases were new introductions of SARS-CoV-2 and not lingering virus from the original outbreak. That helped the Chinese government decide to implement only limited lockdowns and testing of people in specific apartment blocks around a food market where the cluster of cases emerged, rather than resort to a citywide quarantine.
And there are other, less obvious ways that genetic analysis of SARS-CoV-2 could help to predict surges in cases as people emerge from lockdown. Italian scientists have sampled wastewater from sewage treatment plants in northern cities where the pandemic flourished, and found evidence of SARS-CoV-2 weeks before the first cases showed up to flood the hospitals. In La Crosse, Wis., Paraic Kenny, director of the Kabara Cancer Research Institute of the Gundersen Health System, applied the same strategy in his hometown in the spring. A few weeks later, in mid-June, when cases of COVID-19 surged because of bars reopening in downtown La Crosse, Kenny compared samples from infected people with the viral genomes in his wastewater samples. They were a genetic match. The same strain of SARS-CoV-2 had been circulating in the community weeks before the cases were reported. “In principle, an approach like this can be used to not just ascertain how much virus is in the community, but maybe give hospitals and public-health departments a warning of when to anticipate a surge in cases,” he says. The goal is to know not just where we are today but where we will be a week or two from now.
It has been 100 years since an infectious disease pushed the entire world’s population into hiding to the extent that COVID-19 has. And the primary approaches we take to combatting emerging microbes today are likewise centuries old: quarantine, hygiene and social distancing. We may never learn exactly where SARS-CoV-2 came from, and it’s clearly too late to prevent it from becoming a global tragedy. But extraordinary advances in scientific knowledge have given us new tools, like genetic sequencing, for a more comprehensive understanding of this virus than anyone could have imagined even a decade or two ago. These are already providing clues about how emerging viruses like SARS-CoV-2 operate and, most important, how they can be thwarted with more effective drugs and vaccines.
This knowledge can save millions of lives–as long as science leads over politics. As unprecedented as this pandemic seems, in both scope and speed, it shouldn’t have caught the world by surprise. For decades, scientific experts have been warning that emerging zoonotic viruses are a threat to humanity of the greatest magnitude. “People keep using the term unprecedented. I’ll tell you, biologically, there is nothing unprecedented about this virus really,” says Holmes, the evolutionary biologist. “It’s behaving exactly as I would expect a respiratory virus to behave.” It’s simply how viruses work, have always worked and will continue to work. The sooner we accept that, the sooner we can act on that knowledge to control outbreaks more quickly and efficiently.